
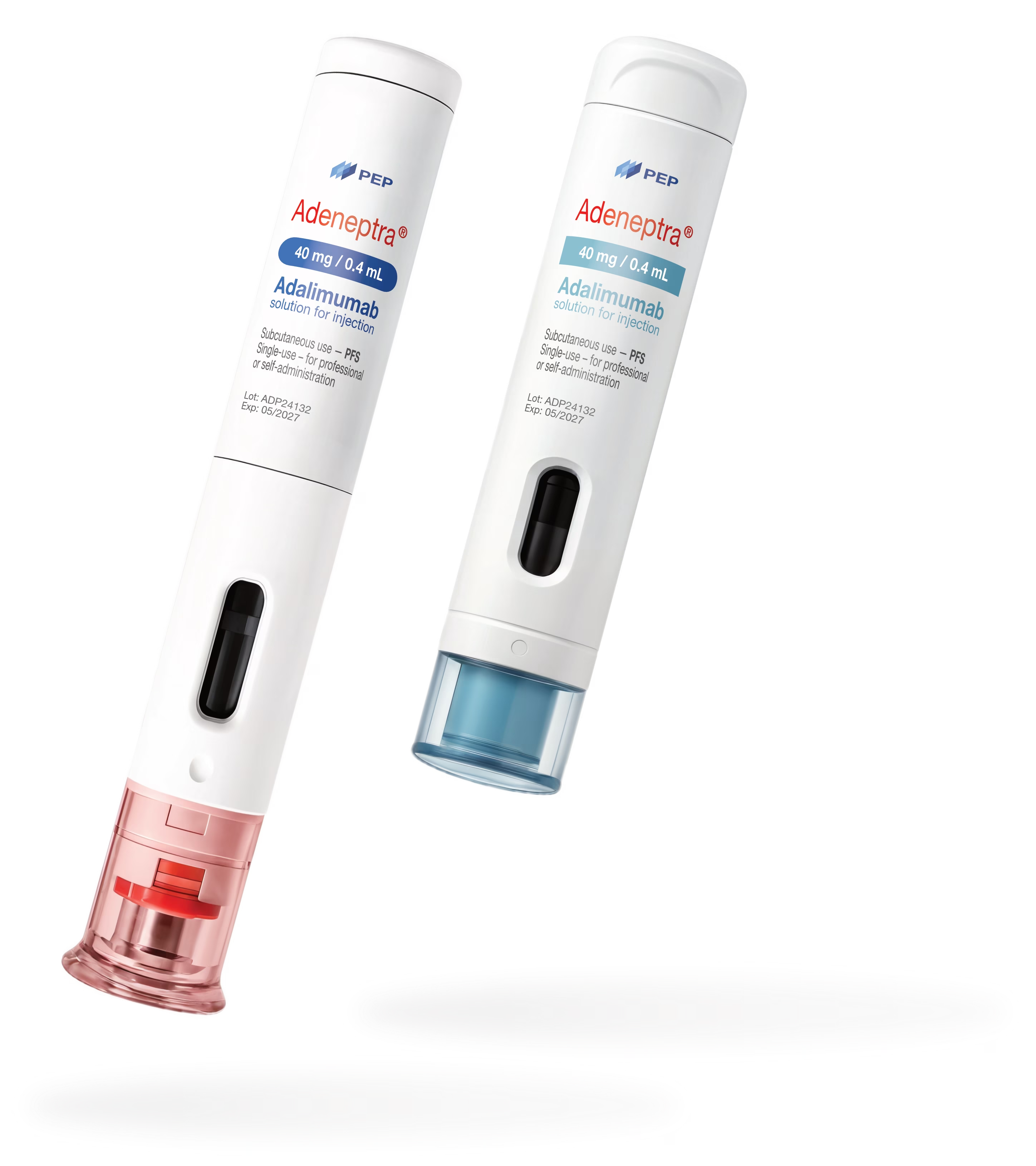
Serialized Rx & OTC
Wrap-around and flag labels pre-printed with GS1 GTIN, random serial, lot & expiry. 100 % vision-inspected for unit-level traceability.
EU FMD
DSCSA
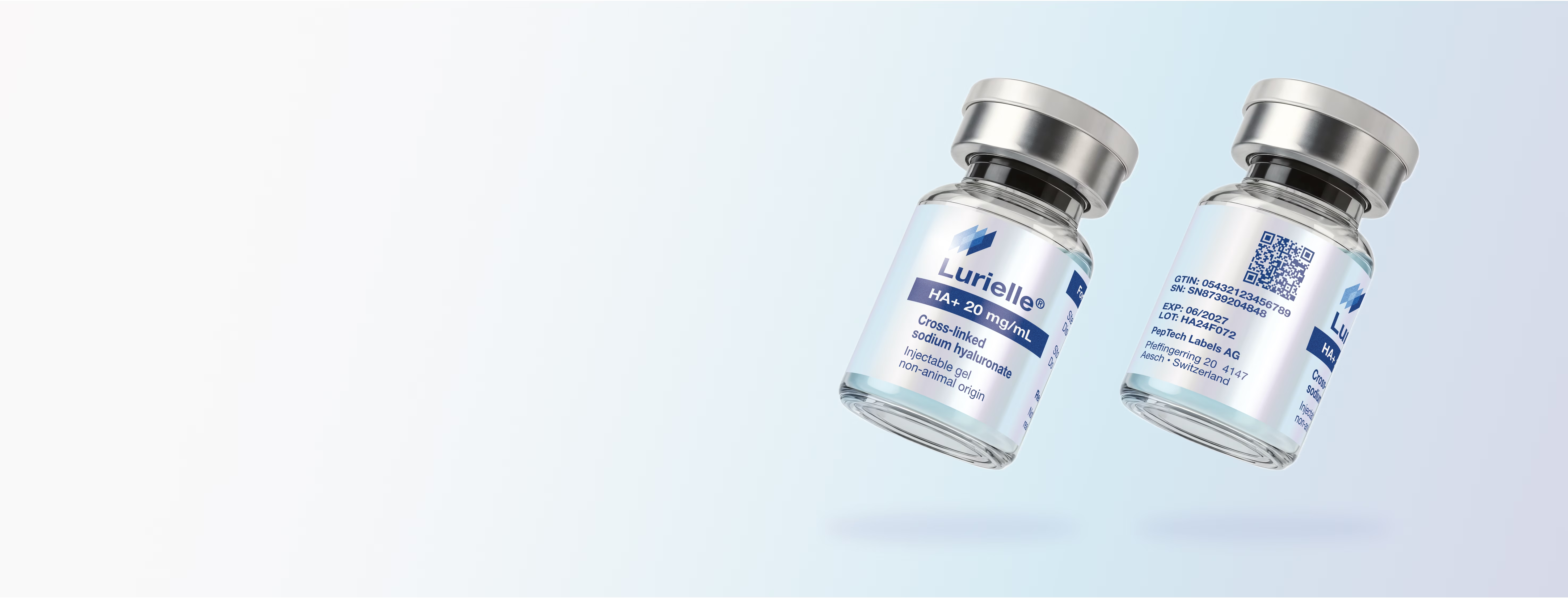
Pharma-grade Labels Audit-ready. On time
Labels for vials, syringes, devices and cold-chain packs, manufactured under GMP-compliant conditions, with processes aligned to the principles of ISO 15378
Complete Label Portfolio
Wrap-around, booklet, cryogenic and tamper-evident labels, engineered adhesives and substrates for every pharmaceutical use case.
Each design is validated for sterility, migration, and print durability, then produced on digital and flexo lines for run sizes from clinical batches to global launches.

Wrap-around and flag labels pre-printed with GS1 GTIN, random serial, lot & expiry. 100 % vision-inspected for unit-level traceability.
EU FMD
DSCSA

Variable-data sets for double-blind, open-label or emergency-unblinding studies, generated under 21 CFR Part 11 digital controls. Tamper-proof over-labels included.
21 CFR §11
GMP

Steam-sterilisable UDI labels for vials, trays and kits; barcodes remain scannable after 60 autoclave cycles.
MDR 2017/745
ISO 13485

Adhesives rated to –196 °C; survive 100 freeze–thaw cycles on vials, bags, plates without flagging or ink bleed.
LN₂
Void, frangible, holographic and UV-reactive layers integrated for diversion control; fully compatible with serialized codes.
ISO 21976

Up to 56-page leaflets or peel-reseal labels for multilingual IFUs on small containers; reseal strength validated to ASTM F88.
EMA QRD
ASTM F88
Service & Supply Reliability
Swiss-run VMI hubs, DNV-audited performance data, and late-stage customisation give supply and QA teams one partner from engineering to palletised labels—99.8 % on-time-in-full across DACH.
ISO 9001
Label Engineering
Material stack-up, adhesive selection, liner choice, bar-code zone and gap tolerances validated to ISO 15378.
Design Validation
Prototype testing and regulatory compliance review for optimal performance.
Production Setup
Digital and flexo printing line configuration with quality controls.
Quality Control
100% vision inspection and compliance verification before delivery.
Delivery & Support
On-time delivery with ongoing technical support and maintenance.
We are part of the global fight against counterfeit medicines
Security & Traceability
Covert UV inks, micro-text, and EU FMD / DSCSA serialisation built in. ISO 21976 tamper seals and audit-ready data blocks diversion at every hand-off.
MICRO-TEXT / GUILLOCHE
2D DATAMATRIX SERIALISATION (EU-FMD/DSCSA)
UV FLUORESCENT INK (COVERT)
ISO 21976 TAMPER-SEAL
AUDIT-READY TRACEABILITY
Key Figures
800 million
labels produced on GMP lines (2024)
10 Days
fastest GMP validated lead-time
-192°C / 90°C
adhesives qualified to LN₂ / autoclave extremes
Regulatory Confidence
100 % in-line inspection, 250 + client audits passed without major findings.
Our documentation pack covers material specs, CoAs, and traceability reports, so your team spends less time preparing for inspections and zero time fixing relabel issues.
Ready for audit-proof labelling?
Talk live with a Pharma Label Advisor—get GMP guidance, timeline options and a compliance checklist in a focused 15-minute call.
ISO 15378 facility · DNV-audited KPIs
Mini FAQ
PepTech Labels AG is certified to ISO 9001:2015 and ISO 14001:2015 by DNV. We operate under GMP- compliant conditions, and our processes are aligned with the principles of ISO 15378 (primary packaging for medicinal products).
Our qualified range spans -196 °C liquid-nitrogen cryo storage to +90 °C steam autoclave, including freeze-thaw cycling and -40 °C cold-chain transit.
Yes. We generate, verify, and inline-print GS1 GTIN, random serial numbers, lot and expiry. A validated vision system records every code and exports upload-ready XML/CSV files.
If required, every order ships with a batch record, material CoA, migration statement, and a camera-inspection report linking each serialised code to roll ID.
Emergency runs ship in 48 hours once artwork is approved and components are in stock; standard IMP batches leave in 5-7 working days.
